How have two elements – critical to life on earth – become the driving force behind the destruction of ecosystems around the globe?
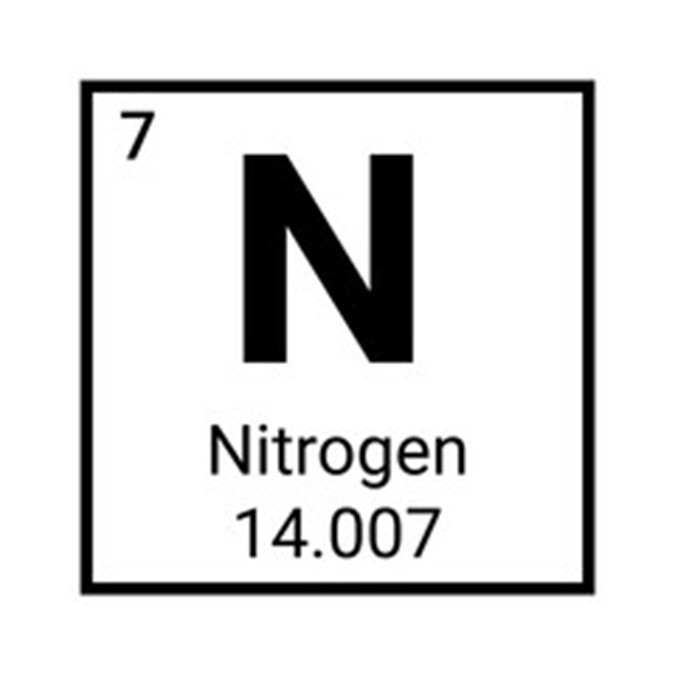
Nitrogen
As one of the primary elements on earth, nitrogen (atomic number 7 on the periodic table) makes up nearly 80% of the earth’s atmosphere. It is also an important component of amino acids, and as such nitrogen is fundamental to all living things. Used in many industrial applications, nitrogen is also significant to the manufacture of a whole host of products, including fertilizer, chemicals, nylon, dyes, and explosives.
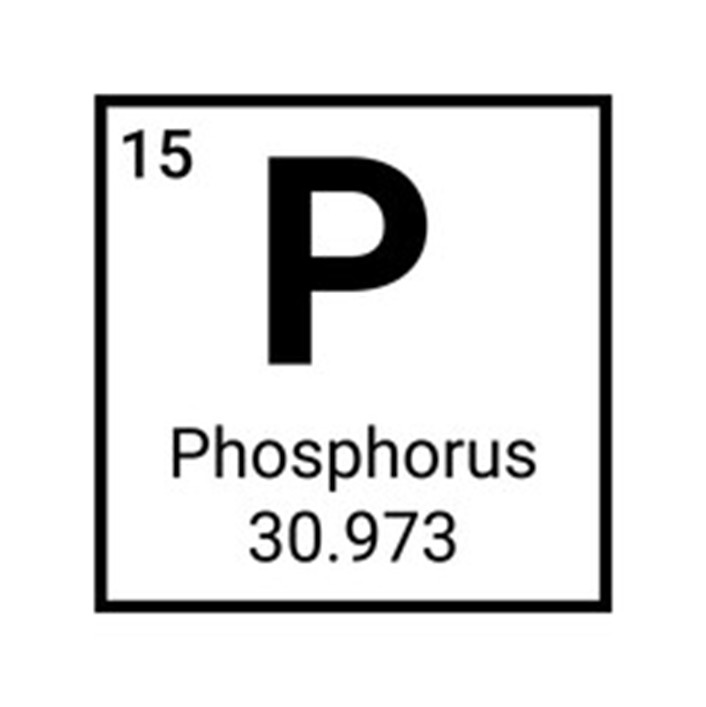
Phosphorus
Phosphorus (atomic number 15), not unlike nitrogen, is also essential to all living things, as it is the backbone of DNA and RNA. In addition to being naturally occurring in all living organisms, calcium phosphate is the primary element in a mammal’s tooth and bone structure. The primary use of manufactured phosphorus is as a fertilizer, developed into ammonium phosphate for soil nutrition to enhance plant growth. As a building block for plant material, it provides fundamental nourishment to starter plants and young seedlings.
So how is it that these two basic elements, providing essential nutrients to life as we know it, causing such turmoil in our environmental ecosystems? It all comes down to nutrient pollution and the adage, “too much of a good thing.”
What is nutrient pollution?
Naturally occurring nutrients – primarily nitrogen and phosphorus – at normal, balanced levels in lakes, rivers and other bodies of water are necessary and healthy, as they support the growth of algae and aquatic plants. These plants in turn provide food and habitat for fish, shellfish, and smaller organisms that live in the water. Too much nitrogen and phosphorus create what is referred to as nutrient pollution.
What is the source of excess nitrogen and phosphorus?
The overabundance of nitrogen and phosphorus typically originates from fertilizer or waste runoff from factory farming, industrial processing facilities and municipal wastewater treatment plants. But it’s not just big business that is adding to nutrient pollution around the globe. Also pervasive in our communities and towns is the excessive use of fertilizers for lawn maintenance and golf courses, to attain the perfect landscaping backdrop for homes and businesses. Excess water runoff during storms funnels directly into local waterways – oceans, lakes, rivers and streams – or to the closest catch basin on the street (which in turn typically feed directly into waterways). No matter the source, however, the runoff brings a level of nutrient pollution that is difficult for any body of water to process or absorb.

Why is nutrient pollution a problem?
Nutrient pollution accelerates the growth of algae and leads to harmful algae blooms (HABs), which deteriorate water quality, reducing or even eliminating the oxygen supply and reducing the exposure to sunlight. Making matters worse, nutrient pollution has been exacerbated by climate change, with increased water temperatures (more conducive to algae growth) and bigger storms (heavier rainfall leading to increased erosion leading to a greater influx of nutrients from the runoff). Nutrient pollution leads to habitat damage, loss of aquatic life, drinking water contaminated with toxins and bacteria, and ultimately negative impacts on human health. According to the New York State (United States) Department of Health, contact with contaminated surface waters can cause diarrhea, nausea, or vomiting; skin, eye, or throat irritation; and allergic reactions or breathing difficulties.
What can be done?
As much as wastewater treatment plants – by removing nitrogen and phosphorus in their processing stream – are a critical solution aimed at addressing nutrient pollution, it is still an all-too-common occurrence in communities around the U.S. and around the globe. It is estimated that the cost of treating and preventing HABs – in the U.S. alone, between the years 2010 and 2020 – cost more than $1 billion, and that figure is expected to rise as global temperatures rise.
If the presence of excess nitrogen and phosphorus in our water remains unchecked, then nutrient pollution will continue to wreak havoc on our ecosystems. And precious, clean water in our bodies of water – from our biggest oceans to our smallest rivers and streams – will continue to be contaminated by these excesses, unless we take a serious look at the root source of these nutrients and begin to make changes that will make a difference.








